There are 7,400 cases of ovarian cancer every year in the UK, one of the highest rates in Europe, and 70% of patients will have a recurrence. Delaying cancer growth for as long as possible is integral, and now a ‘game changer’ drug has been approved for use on the NHS that could do just that.
Niraparib showed a delay in cancer growth by around six to 15.5 months more than a placebo, according to clinical trials, depending on a woman’s genetic profile. The drug was previously not included in the Cancer Drugs Fund, meaning many women didn’t have access to it.
However, after being approved by the National Institute for Health and Care Excellence, the drug will be made available to women with recurrent, platinum-sensitive ovarian, fallopian tube or peritoneal cancer who have had two or more courses of platinum-based chemotherapy.
Up to 850 women per year could benefit from the drug, which is taken once a day and works by inhibiting two proteins involved in DNA repair to prevent cancer growth. However, while clinical trials did show a delay in cancer growth, the final results on overall survival were not available, so it’s unclear as to whether Niraparib increases the length of time people live.
Yet, the drug has been dubbed a ‘game changer’ by experts. Rebecca Rennison, director of public affairs and services at Target Ovarian Cancer, said:
‘Today’s announcement is a game changer in ovarian cancer. While we have seen some new treatments in recent years, these have been for highly restricted groups.
'With Niraparib, we’re taking the fight to ovarian cancer. We know that with the right investment in new treatments, more women can and will survive this disease. Today is a critical first step in making that a reality.'
The inclusion and further use also means that doubts about the drugs success rate can be addressed. Meindert Boysen, director of the centre for health technology evaluation at Nice, said:
‘The outcome for women with ovarian cancer is generally poor, with less than 35% surviving for five years after diagnosis.
‘We are pleased to see the inclusion of niraparib in the Cancer Drugs Fund as it will give women early access to this treatment while uncertainties in the clinical evidence can be addressed through the collection of additional data.'
This is the first time a breakthrough drug has been made available on a mass scale for women with recurrent ovarian cancer, meaning the drug's approval is being seen as a milestone moment for ovarian cancer treatment.
Click through to see facts about women around the world...
Debrief Facts about women around the world
 1 of 18
1 of 18Facts about women around the world
 2 of 18
2 of 18Facts about women around the world
 3 of 18
3 of 18Facts about women around the world
 4 of 18
4 of 18Facts about women around the world
 5 of 18
5 of 18Facts about women around the world
 6 of 18
6 of 18Facts about women around the world
 7 of 18
7 of 18Facts about women around the world
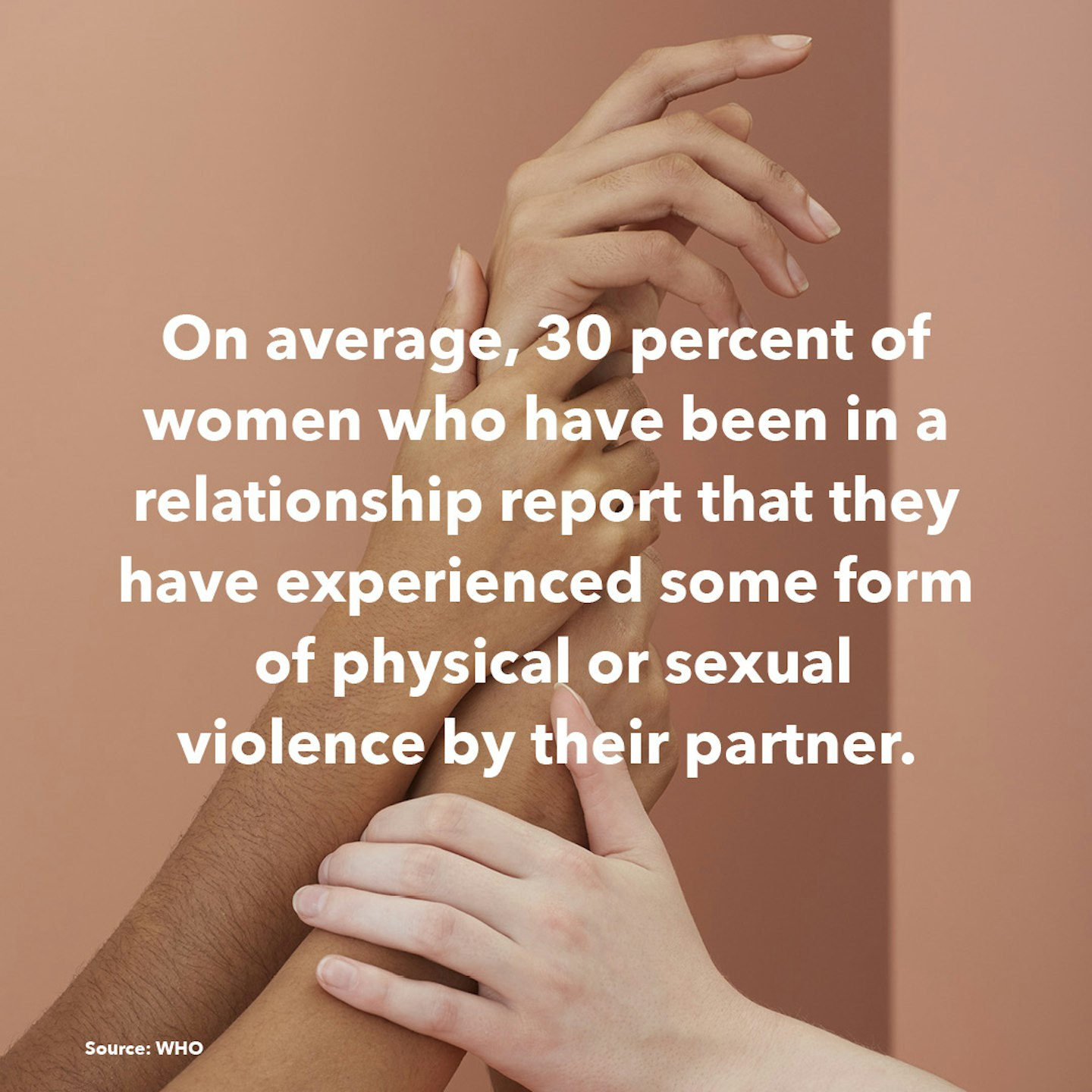 8 of 18
8 of 18Facts about women around the world
 9 of 18
9 of 18Facts about women around the world
 10 of 18
10 of 18Facts about women around the world
 11 of 18
11 of 18Facts about women around the world
 12 of 18
12 of 18Facts about women around the world
 13 of 18
13 of 18Facts about women around the world
 14 of 18
14 of 18Facts about women around the world
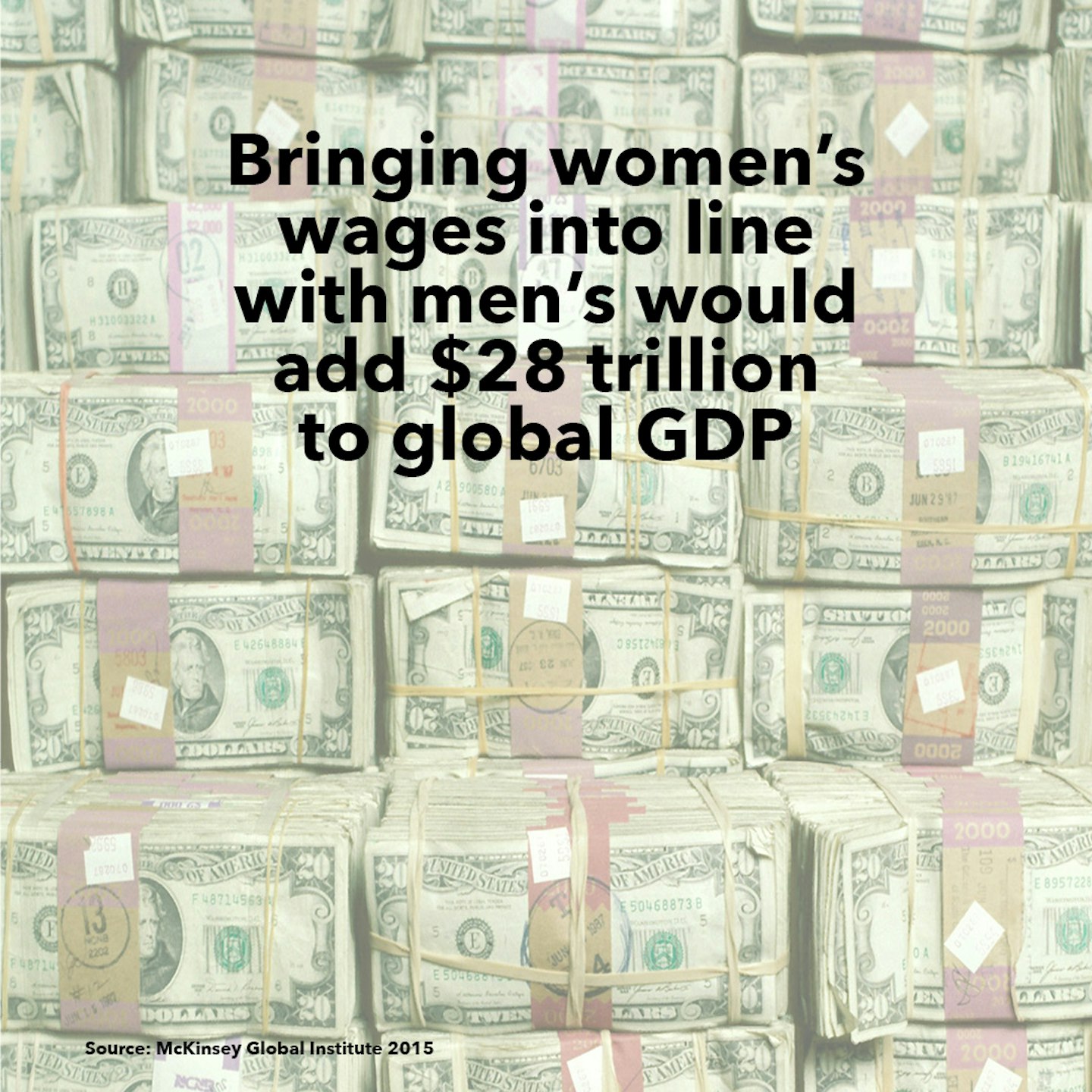 15 of 18
15 of 18Facts about women around the world
 16 of 18
16 of 18Facts about women around the world
 17 of 18
17 of 18Facts about women around the world
 18 of 18
18 of 18